Phytophthora cambivora -
Species description:
The genus Phytophthora according to the current systematic classification, belongs to the kingdom Chromista, phylum Oomycota, order Peronosporales, family Peronosporaceae. Thus, they are not fungi in the true sense, but they will be named from a practical and historical point of view in the following text.
It is a microscopic fungus from which only the mycelium is visible to the naked eye, even after cultivation of the sample in a humid chamber. All other morphological structures of the fungus are visible only under a binocular magnifier (magnification 10-25x) or a microscope (magnification 100-400x).
The fungus forms the following morphological structures:
• asexual: sporangia, zoospores, chlamydospores
• sexual: oospores, antheridia, oogonia
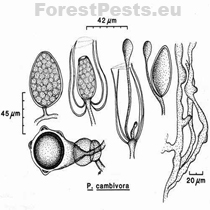
Sporangia: are broad-ovate, almost spherical, obpyriform to elliptical with a rounded base. Sporangia are non-papillate and thus belong to group VI. They are formed by growing on the lateral mycelium or at the end of the main mycelium. After releasing zoospores from the sporangium, a new sporangium is formed within the just emptied. Sporangiophores have simple growth, are unbranched, or branching is sympodial. The size of the spore is 40-54x60-75 µm.
Chlamydospores are formed very rarely.
Oospores, oogonia, and antheridia form the sexual structures. The fungus is heterothallic, i.e., two different "genders" named in this text "pairing types A1 and A2". Only when the mycelia of opposite pairing types merge the formation of oospores occurs. Sometimes, type A2 isolates form oogonia even in pure monoculture without the opposite pairing type A1.
The antheridia ♂ are amphigenic, i.e., the connection with the oogonia occurs by overlapping the female ♀ oogonia part with the male ♂ antheridium. The size of the antheridia is 14-32x12-21µm (with an average of 19x16 µm), so they are primarily oval, single-celled, or double-celled.
Oogonia ♀ they are spherical; more than 50% of the surface is covered with balls or bumps. Oogonia form after the mycelium mate with the opposite type of the same species of P. cambivora or another species (e.g., P. cinnamomi or P. cryptogea). The oogonia diameter is 30-58 µm (with an average of 48 µm). The wall thickness of the oogonia is up to 2 µm. In the end, hyphae form.
The oospores are plerotic (i.e., there is no free space between the oospore and the oogonium, the oospore fills the entire oogonium). The diameter size of the oospores is 37-44 µm (with an average of 40 µm). The thickness of the oospore wall is up to 3 µm.
The mycelium of the fungus grows at a temperature of 2 to 32 ° C with an optimum of 22 to 24 ° C. The mycelium is coral, sometimes twisted into irregular shapes, with a slight to abundant growth of airy mycelium. Older mycelium can be compartmented! The pure culture sample on the nutrient medium can be star-shaped to monolithic.
It differs from Phytophthora cinnamomi in that P. cambivora:
• rarely forms slightly pigmented mycelium,
• grape, thin-walled mycelial swelling,
• hardly forms chlamydospores at all,
• oogonia are bumpy to spherical on the surface.
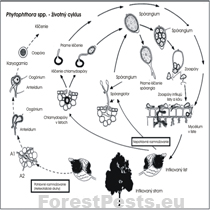
Bionomy - infection cycle:
Phytophthora cambivora is a pathogen that uses soil as an environment for growth, development, and reproduction, a so-called soil pathogen. The disease in the infected root or trunk develops quickly when the increased soil moisture remains longer, and the temperature does not exceed 30 ° C.
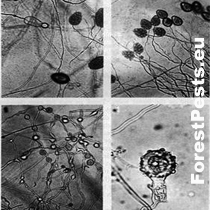
Figure 3. Papilla-free sporangia, oogonium with antheridium and oospore, sporangia proliferation (mycelium overgrowths through empty sporangium) Photo:http://www.phytophthoradb.org/file/html_fppd/phytophthora/cambivora/Figure_1.htm)
 |
 |
 |
|
The life cycle of fungi of the genus Phytophthora (heterothallic species) |
Two photos above - sporangia, two photos below Oogonia with amphigenic antheridium, distinct plerotic oospore. Photo: http://www.phytophthoradb.org/file/html_fppd/phytophthora/cambivora/Figure_2.htm)
|
Papilla-free sporangia, oogonium with antheridium and oospore, sporangia proliferation (mycelium overgrowths through empty sporangium)
html_fppd/phytophthora/cambivora/
Figure_1.htm)
|
Symptom:
The fungus causes infection of the roots and root necks of various forest and fruit woody plants. However, it is known mainly for the so-called Ink disease of chestnut. P. cambivora infects the chestnut tree at the base of the trunk and the coarser roots. Root and trunk rot can develop slowly or can be rapid. If the fungus circles around the trunk at its base, the whole tree crown withers, and the tree dies within one year. If the infection develops slowly, the symptoms of the infection are a reduction of leaves and fruits size in the first year of infection, and a tree usually dies in the second year after infection. Although the disease is named after the ink exudate from the trunk base, this symptom is rare. The infested roots also secrete this ink exudate, and the soil around such roots is colored. A similar disease is caused by Phytophthora cinnamomi.
Endangered woody plants:
It attacks woody plants of the genus Prunus, Malus, and forest woody plants, e.g., Castanea sativa, Fagus sylvatica, etc.
In Slovakia, its occurrence is known on Castanea sativa; the first finding is from 1972.
Origin and distribution:
The fungus comes from tropical and subtropical regions, especially from Australia. Climate warming creates suitable conditions for the life of this fungus also in Slovakia, especially in the southern areas, where the winter is mild.
Endangered stands and areas:
Beech stands are particularly endangered (31.6% forests of cover, 632 thousand ha). The pathogen can be transmitted to the forest from gardens where ornamental woody plants died (e.g., rhododendrons, etc.) and were carried as a waste to the forest "where they later rot". Thus, the first diebacks can be observed next to houses and residential areas in the woods. This is because gardens are intensively maintained - dead plants are replaced with new ones, mainly bought in gardening supplies, where plants are imported from abroad and from regions where the disease is common.
Prevention:
Plant seedlings in the disease-free stand. It is necessary to prepare a functional system (protocol) of plant growing hygiene in the production of seedlings in nurseries and ensure strict compliance.
Select resistant tree species or their cultivars (if they have been bred) for planting.
Keep the soil free of infectious structures. Among the chemical substances, metalaxyl, salts of phosphoric acid (e.g., fosetyl-Al, and the others), or phenylamide show biological (fungicidal and fungistatic) efficiency.
Among biologically active organisms, fungi's antagonistic properties from Trichoderma and Gliocladium genera are effective.
The water used for watering and irrigating must be disinfected, e.g., chlorine.
Monitoring:
Usually, from the stump up to about 2 m in the height of the trunk, tar-like stains are searched. These spots usually occur on the tree, showing chlorosis, small leaves, leaves necrosis, and defoliation. Below the bark periderm, there are apparent changes in the color of the tissues. Next, a sample of about 2 cm is taken to the laboratory to isolate the pathogen. The final determination depends on the microscopic properties of sporangia, zoospores, oogonia, antheridia, and oospores. Genetic analyzes are also used, but usually, the pathogen must be isolated in pure culture, and to start the analysis, it is necessary to have multiple samples collected (due to the economics of the analysis). Trees of all ages can be attacked, from seedlings to centuries-old trees.
Pest control:
Methods of defense against chestnut infection include:
• sanitation, which includes maintaining seedlings and soil in nurseries without infectious structures,
• growing forest tree seedlings and trees in well-drained seed-beds and areas,
• if possible, grow resistant trees (Castanea crenata) or resistant cultivars,
• removal of bark from infested trees with subsequent treatment of occurred injuries and soil with copper fungicides, metalaxyl or fosetyl-Al. Copper fungicides slow the rate of infection spread if they are applied in the early stages of the disease. Metalaxyl applied to the soil slows the root and trunk bark necrosis formation. Fosetyl-Al is applied to assimilation organs but does not affect the soil.
Pest category: Fungi
Found in Slovakia: Yes
Invasive species: Yes
Similar species: All species of the genus Phytophthora, and also Pythium.
Literature:
Bernadovičová, S., Juhásová, G., 2005: Cultura characteristics of Phytophthora fungi – causal agent of ink disease on chestnut trees (Castanea sativa Mill.) in Slovakia. Folia Oecologica 32 (2): 51-58.
Bharath, S., Phillips W., Krauss, U., 1999: Practical Notes on Work with Phytophthora Species. In: Krauss, U. and Hebbar, P., Research Methodology in Biocontrol of Plant Diseases with Special Reference to Fungal Diseases of Cocoa, Workshop Manual, CATIE, Turrialba, Costa Rica, p.123-130.
Brasier, C. M., Rose, J., Gibbs, J. N., 1995: An unusual Phytophthora associated with widespread alder mortality in Britain. Plant Pathology, 44: 999-1007.
Brasier, C., 2007: Phytophthora Biodiversity: How Many Phytophthora Species Are There? In: Proceedings from the Forth Meeting of IUFRO Working Party S07.02.09, Phytophthoras in Forests and Natural Ecosystems, August 26 – 31, 2007, Monterey, California, p. 101 – 115.
Day, W. R., 1938: Root-rot of sweet chestnut and beech caused by species of Phytophthora. I. Cause and symptoms of disease: its relation to soil conditions. Forestry, Oxford, 12: 101-116.
Day, W. R., 1939: Root-rot of sweet chestnut and beech caused by species of Phytophthora. II. Inoculation experiments and methods of control. Forestry, Oxford, 13: 46-58.
Doster, M.A., Bostock, R.M., 1988: Susceptibility of almond cultivars and stone fruit species to pruning wound cankers caused by Phytophthora syringae. Plant Disease, 72: 490-492.
El-Hamalawi, Z.A., Menge, J.A., 1994: Effects of wound age and fungicide treatment of wounds on susceptibility of avocado stems to infection by Phytophthora citricola. Plant Disease, 78: 700-704.
Erwin, D.C., Ribeiro, O.K., 1996: Phytophthora Disease Worldwide. APS Press, St. Paul, Minnesota, 562 pp.
Gibbs, J. N., 1995: Phytophthora Root Disease of Alder in Britain. EPPO Bulletin, 6 pp.
Gibbs, J., 1994: Phytophthora Root Disease of Common Alder. Forestry Commission, Research Information Note, 258, 4 pp.
Gibbs, J., Lonsdale, D., 1996: Phytophthora Disease of Alder: the situation in 1995. Forestry Commission, Research Information Note, 277, 4 pp.
Hartmann, G., Blank, R., 1998: Buchensterben auf zeiweise nassen Standorten unter Beteiligung von Phytophthora-Wurzalfäule. Forst und Holz, 53 (7): 187-193.
Hartmann, G., Blank, R., 2002: Vorkommen und Standortbezüge von Phytophthora-Arten in geschädigten Eichenbeständen in Nordwestdeutschland (Niedersachsen, Nordrhein-Westfalen und Schleswig-Holstein). Forst und Holz, 57 (18): 539-545.
Juhásová, G., 1999: Hubové choroby gaštana jedlého (Castanea sativa Mill.). Veda, Bratislava, 191 pp.
Juhásová, G., Satko, J., 1997: Zdravotný stav Castanea sativa Mill., výskyt huby Cryphonectria parasitica (Murr.)Barr a možnosti biologickej ochrany na Slovensku. In: Pestovanie a ochrana gaštana jedlého (Castanea sativa Mill.) na Slovensku, Zborník, Ústav ekológie lesa SAV Zvolen, Pobočka biológie drevín Nitra, Nitra, 17 - 35.
Jung, T., Blaschke, H., 1995: Phytophthora root rot in declining forest trees. Phyton (Horn, Austria), 36 (3): 95-102.
Jung, T., Blaschke, H., Neumann, P., 1996: Isolation, identification and pathogenecity of Phytophthora species from declining oak stands. Eur. J. For. Path., 26: 253-272.
Jung, T., Cooke, D. E. L., Blaschke, H., Dunkan, J. M., Oßwald, W., 1999: Phytophthora quercina sp. nov., causing root rot of European oaks. Mycol. Res., 103 (7): 785-798.
Jung, T., Hansen, E. M., Winton, L., Oßwald, W., Delatour, C., 2002: Three new species of Phytophthora from European oak forests. Mycol. Res., 106 (4): 397-411.
Kunca, A., 2003: Phytophthora ako vážny patogén buka lesného v Nemecku. In. Varínsky, J. (Ed.), Zborník referátov z celoslovenského seminára Aktuálne problémy v ochrane lesa 2003, Banská Štiavnica, 24-25.4.2003, p. 175-178.
Lilja, A., Rikala, R., Hietala, A., Heinonen, R., 1996: Stem lesions on Betula pendula seedlings in Finnish forest nurseries and the pathogenecity of Phytophthora cactorum. Eur. J. For. Path., 26: 89-96.
Mitchell, D.J., Kannwischer-Mitchell, M.E., 1992: Phytophthora. In: Singleton, L.L., Mihail, J.D., Rush, C.M. (eds), Methods for Research on Soilborn Phytopathogenic Fungi, APS Press, St. Paul, Minnesota, p. 31-38.
Robin, C., Smith, I., Hansen, E.M. 2012. Phythophthora cinnamomi. Forest Phytophthoras 2(1). doi: 10.5399/osu/fp.2.1.3041
Sánchez, M. E., Caetano, P., Ferraz, J., Trapero, A., 2002: Phytophthora Disease of Quercus ilex in south-western Spain. Forest Pathology, 32 (1): 5-18.



